phosphorus valence electrons
The first is to use the Periodic Table to figure out how many electrons Phosph. 5 rows How many electrons does phosphorus have.
 |
| Based On The Diagram Describe The Atomic Structure Of Phosphorus Be Sure To Include In Your Brainly Com |
In the PCl5 molecule the central phosphorus atom is bonded to.
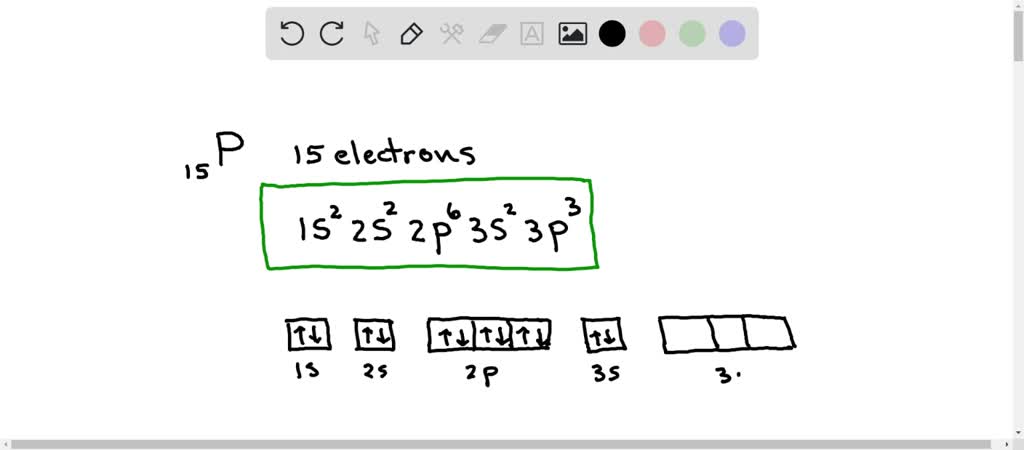
. In the compound PCl5 phosphorus has 10 valence electrons. Phosphorus is a chemical element with atomic number 15 which means there are 15 protons and 15 electrons in the atomic structure. Phosphorus-33 is composed of 15 protons 18 neutrons and 15 electrons. 2 Using Electron Configuration First write electron configuration of phosphorus The electron configuration of.
And the total number of electrons present in this energy level is 2. You can see in the electron configuration of phosphorus 1s2 2s2 2p6 3s2 3p3 that the highest energy level is 3. 6 rows The number of valence electrons available for the Phosphorus atom is 5. Phosphorus is an element which is part of Group 15 formally known as the Pnictogen group and is directly below the nitrogen atom.
Its symbol is P. Phosphorus is the element in group 15. Well in simple and straightforward words Phosphorus has five valence electrons. How Many Valence Electrons Does Phosphorus Have.
33P a beta-emitter 025 MeV with a half-life of 254 days. So one can either add 3 electrons to the outer orbit or take away 5 electrons with equal ease. 15 has its electrons arranged in a configuration of 285. How many valence electrons does the phosphate ion have.
They help in the formation of chemical. The chemical symbol for Phosphorus is P. Wiki User 2012-12-06 041319 Study now See answer 1 Copy 32 PO4-3 Phosphate has 5 valence electrons. This article explains in detail the valence electrons for.
How Many Valence Electrons Does PCl3 HaveWhat is the number of valence electrons in PCl3How many valence electrons are in a phosphorus trichloride PCl3. As was mentioned before a neutral. It is used in life-science laboratories in applications in. One can also reckon it as the.
119 rows Valence electrons. Since phosphorus is in group 15 it has 5 valence electrons. There are two ways to find the number of valence electrons in Phosphorous P. What is the symbol for.
Through its valence electrons phosphate forms bonds. Phosphorous is one of the very reactive elements Valence electrons are defined as the electrons present in the outermost electron shell of any element.
 |
| The Number Of Unpaired Valence Electrons In An Atom Of Phosphorus Is |
 |
| Doping N And P Semiconductors Fundamentals Semiconductor Technology From A To Z Halbleiter Org |
 |
| Electron Configuration For Sulfur S |
 |
| Elemental Phosphorus And Electromagnetic Radiation Serrano Ruiz 2014 European Journal Of Inorganic Chemistry Wiley Online Library |
 |
| How Does Phosphorus Form 5 Covalent Bonds The Unconditional Guru |
Posting Komentar untuk "phosphorus valence electrons"